
QUALITY
Clinical Trials
- Catégorie(s) :无分类内容
- Date de publication :2020-12-30 11:38:33
- Visites : 0
As a responsible international pharmaceutical enterprise, Holley-Cotec has always focued to clinical research and application of medicines, and has established long-term cooperation with reputed international scientific research institutions and CRO enterprises. In collaboration with the Oxford University Tropical Medicine Research Units, for global multi-center clinical projects. Published several valued papers in international academic journals of tropical diseases, causing dihydroartemisinin piperaquine phosphate to gain extensive attention in the industry.
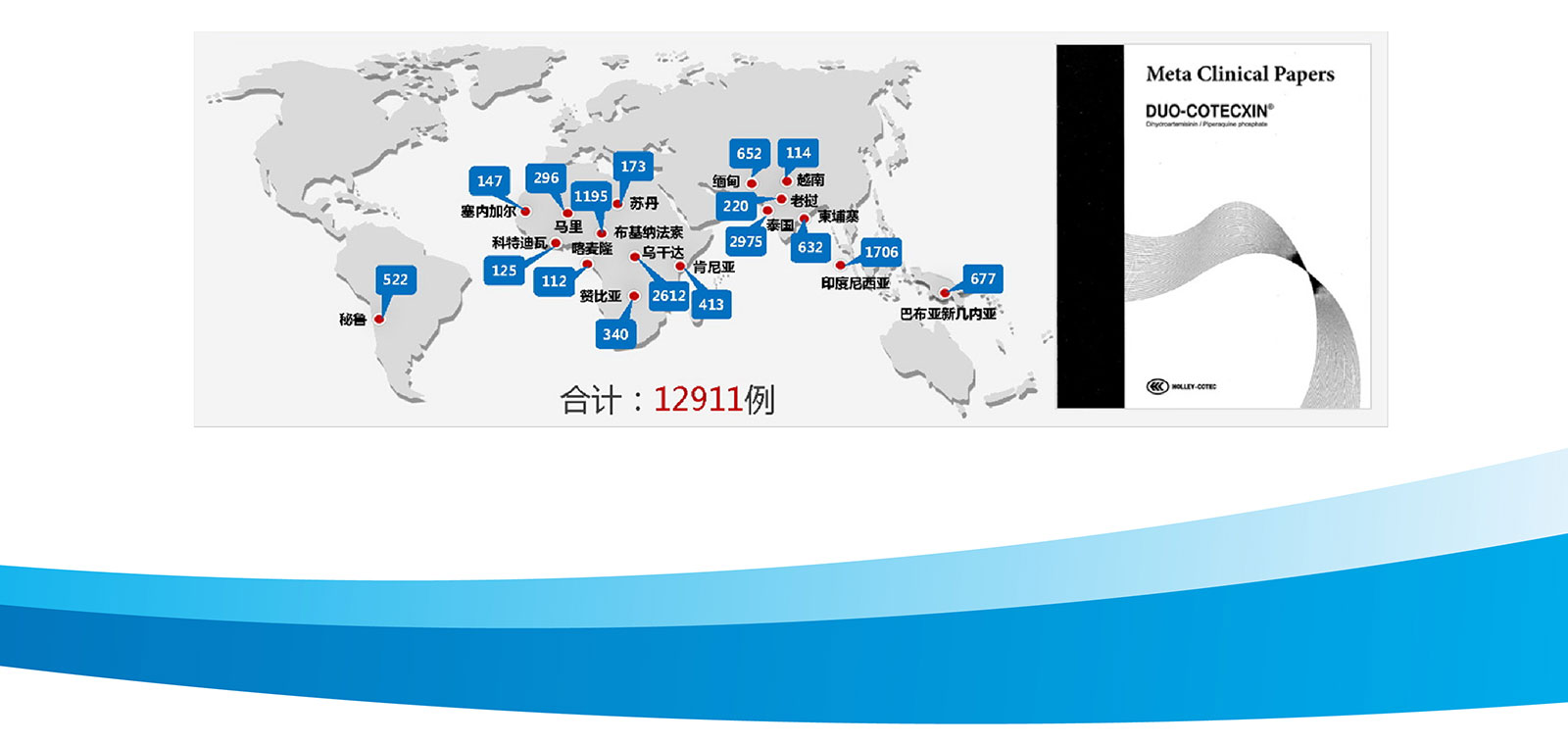
In order to continuously verify the safety and effectiveness of our products, the company carried out rigorous clinical trials in different countries and ethnic groups. These clinical data formed reports and medical literature, which are accepted by many tropical countries and unanimously recognized by the academic community. And therefore dihydroartemisinin entered the malaria medication guidance directory in many countries.
DUO-COTECXIN - Clinical Trails to validate efficacy
In order to continuously verify the safety and effectiveness of the product, rigorous clinical projects have been carried out in multiple countries and different ethnic groups, with a total of 12911 cases.
Scannez le code à l’aide de votre mobile.


 facebook
facebook
 linkedin
linkedin